Page 3 - Dr Stephanie Seneff - Reviewing Some Possible Unintended Consequences of the mRNA Vaccines Against COVID - 19
P. 3
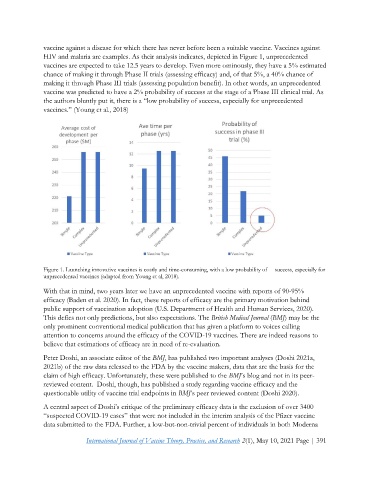
vaccine against a disease for which there has never before been a suitable vaccine. Vaccines against
HIV and malaria are examples. As their analysis indicates, depicted in Figure 1, unprecedented
vaccines are expected to take 12.5 years to develop. Even more ominously, they have a 5% estimated
chance of making it through Phase II trials (assessing efficacy) and, of that 5%, a 40% chance of
making it through Phase III trials (assessing population benefit). In other words, an unprecedented
vaccine was predicted to have a 2% probability of success at the stage of a Phase III clinical trial. As
the authors bluntly put it, there is a “low probability of success, especially for unprecedented
vaccines.” (Young et al., 2018)
Figure 1. Launching innovative vaccines is costly and time-consuming, with a low probability of success, especially for
unprecedented vaccines (adapted from Young et al, 2018).
With that in mind, two years later we have an unprecedented vaccine with reports of 90-95%
efficacy (Baden et al. 2020). In fact, these reports of efficacy are the primary motivation behind
public support of vaccination adoption (U.S. Department of Health and Human Services, 2020).
This defies not only predictions, but also expectations. The British Medical Journal (BMJ) may be the
only prominent conventional medical publication that has given a platform to voices calling
attention to concerns around the efficacy of the COVID-19 vaccines. There are indeed reasons to
believe that estimations of efficacy are in need of re-evaluation.
Peter Doshi, an associate editor of the BMJ, has published two important analyses (Doshi 2021a,
2021b) of the raw data released to the FDA by the vaccine makers, data that are the basis for the
claim of high efficacy. Unfortunately, these were published to the BMJ’s blog and not in its peer-
reviewed content. Doshi, though, has published a study regarding vaccine efficacy and the
questionable utility of vaccine trial endpoints in BMJ’s peer reviewed content (Doshi 2020).
A central aspect of Doshi’s critique of the preliminary efficacy data is the exclusion of over 3400
“suspected COVID-19 cases” that were not included in the interim analysis of the Pfizer vaccine
data submitted to the FDA. Further, a low-but-non-trivial percent of individuals in both Moderna
International Journal of Vaccine Theory, Practice, and Research 2(1), May 10, 2021 Page | 391