Page 21 - Dr Stephanie Seneff - Reviewing Some Possible Unintended Consequences of the mRNA Vaccines Against COVID - 19
P. 21
Spike Protein Toxicity
The picture is now emerging that SARS-CoV-2 has serious effects on the vasculature in multiple
organs, including the brain vasculature. As mentioned earlier, the spike protein facilitates entry of
the virus into a host cell by binding to ACE2 in the plasma membrane. ACE2 is a type I integral
membrane protein that cleaves angiotensin II into angiotensin(1-7), thus clearing angiotensin II and
lowering blood pressure. In a series of papers, Yuichiro Suzuki in collaboration with other authors
presented a strong argument that the spike protein by itself can cause a signaling response in the
vasculature with potentially widespread consequences (Suzuki, 2020; Suzuki et al., 2020; Suzuki et al.,
2021; Suzuki and Gychka, 2021). These authors observed that, in severe cases of COVID-19, SARS-
CoV-2 causes significant morphological changes to the pulmonary vasculature. Post-mortem analysis
of the lungs of patients who died from COVID-19 revealed histological features showing vascular
wall thickening, mainly due to hypertrophy of the tunica media. Enlarged smooth muscle cells had
become rounded, with swollen nuclei and cytoplasmic vacuoles (Suzuki et al., 2020). Furthermore,
they showed that exposure of cultured human pulmonary artery smooth muscle cells to the SARS-
CoV-2 spike protein S1 subunit was sufficient to promote cell signaling without the rest of the virus
components.
Follow-on papers (Suzuki et al., 2021,
Suzuki and Gychka, 2021) showed that
the spike protein S1 subunit
suppresses ACE2, causing a condition
resembling pulmonary arterial
hypertension (PAH), a severe lung
disease with very high mortality. Their
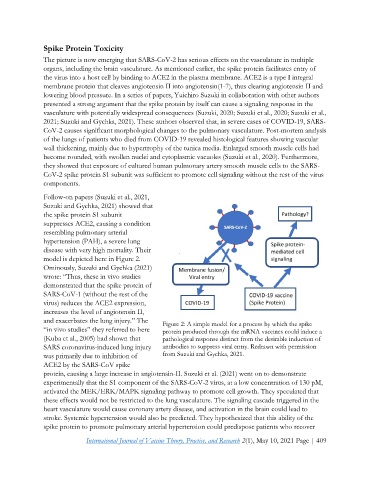
model is depicted here in Figure 2.
Ominously, Suzuki and Gychka (2021)
wrote: “Thus, these in vivo studies
demonstrated that the spike protein of
SARS-CoV-1 (without the rest of the
virus) reduces the ACE2 expression,
increases the level of angiotensin II,
and exacerbates the lung injury.” The Figure 2: A simple model for a process by which the spike
“in vivo studies” they referred to here protein produced through the mRNA vaccines could induce a
(Kuba et al., 2005) had shown that pathological response distinct from the desirable induction of
SARS coronavirus-induced lung injury antibodies to suppress viral entry. Redrawn with permission
was primarily due to inhibition of from Suzuki and Gychka, 2021.
ACE2 by the SARS-CoV spike
protein, causing a large increase in angiotensin-II. Suzuki et al. (2021) went on to demonstrate
experimentally that the S1 component of the SARS-CoV-2 virus, at a low concentration of 130 pM,
activated the MEK/ERK/MAPK signaling pathway to promote cell growth. They speculated that
these effects would not be restricted to the lung vasculature. The signaling cascade triggered in the
heart vasculature would cause coronary artery disease, and activation in the brain could lead to
stroke. Systemic hypertension would also be predicted. They hypothesized that this ability of the
spike protein to promote pulmonary arterial hypertension could predispose patients who recover
International Journal of Vaccine Theory, Practice, and Research 2(1), May 10, 2021 Page | 409